- Form: White powder of 1 mm or less in size
- Composition: Insolubilized and solidified material composed mainly of magnesium oxide (MgO) made from natural magnesite (magnesium carbonate) calcined at approximately 800°C.
What is STA-M?

STA-M Characteristics
- Demonstrates an insolubilizing and solidifying effect via reaction of MgO with added inorganic elements.
- Capable of insolubilization of regulated heavy metals by adding inorganic material to the main MgO material.
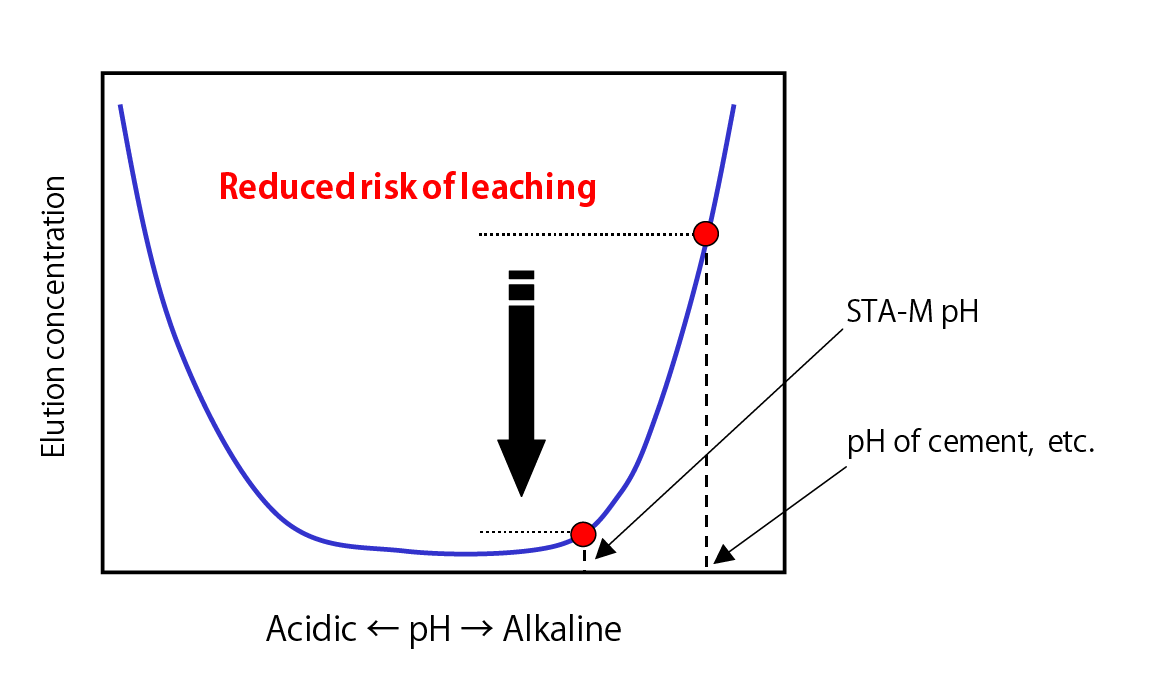
- Material does not contain any calcium-based material so that the post-treatment pH is 10.5 or less, which means that using this material does not cause high alkalinity issues.
- Large specific surface area for good reactivity with soil particles and heavy metal ions.
Magnesium Oxide is the Main Component
| Main component | (%) | Trace ingredients | (ppm) |
|---|---|---|---|
| MgO | 92.13 | Cd | 0.05 |
| CaO | 1.96 | Pb | 0.6 |
| SiO2 | 1.63 | T-Cr | ND |
| Fe2O3 | 0.52 | T-Hg | ND |
| Al2O3 | 0.25 | As | ND |
| SO3 | 0.10 | Cu | 0.7 |
| Igloss | 3.12 | Zn | 7.4 |
| Specific surface area = 4,000 to 12,000 cm2/g | |||
- pH is low in comparison to cement-based solidifiers, which minimizes risk of heavy metal re-dissolution.
Conceptual Diagram Illustrating pH Dependency of Elution Concentration of Lead Compound Ions, etc.
Comparison of Insolubilization Effects on Contaminated Soil
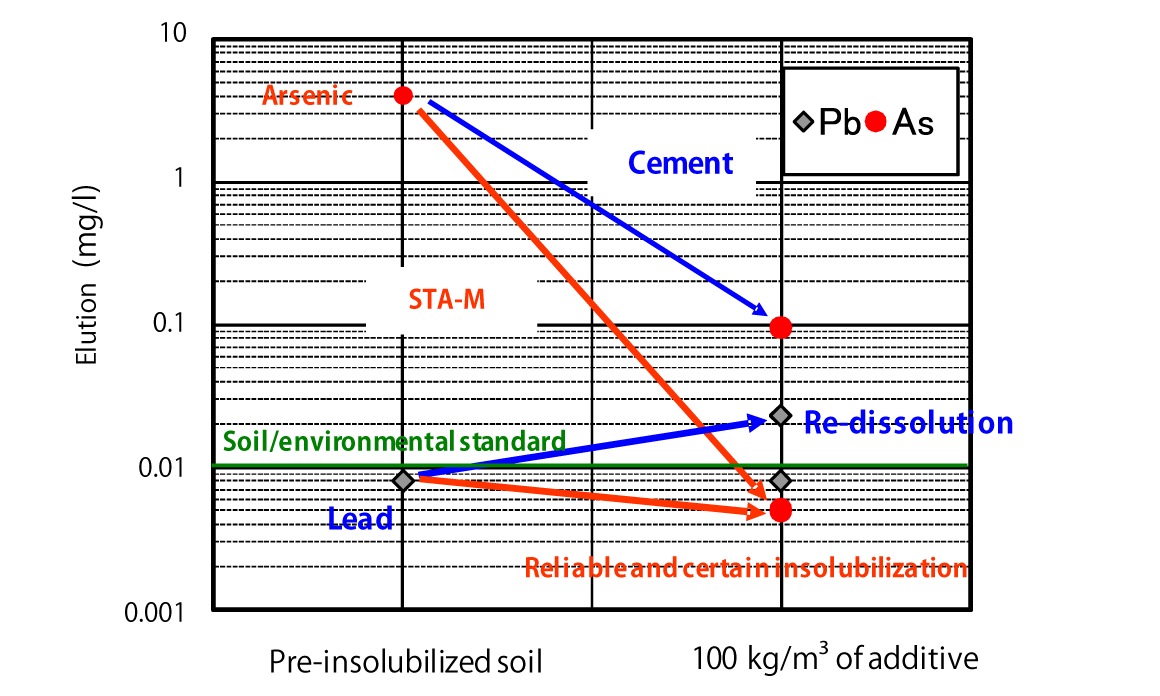
*The provided data is the result of laboratory testing performed according to internal testing methods. Corresponding performance at actual sites is not guaranteed.
- The ability to regulate pH and insolubilization effect of heavy metals can be increased by using this material together with mineral salts.
- Combination with ferrous sulfate improves efficiency of insolubilizing highly contaminated soil.
Initial Pollution Level
| As | Content (mg/kg) | 1,530 |
|---|---|---|
| Elution (mg/L) | 3.8 |
Comparison of Post-treatment Pollution Levels
| Insolubilizing agent Solidifying agent |
Additive (kg/m3) |
Arsenic elution (mg/L) |
qU7 kN/m2 |
pH |
|---|---|---|---|---|
| STA-M | 150 | 0.046 | 1,625 | 9.7 |
| B-type blast furnace | 150 | 1.0 | 2,534 | 10.7 |
| 200 | 0.61 | 3,211 | 11.2 | |
| Ferrous sulfate + B-type blast furnace |
150 | 0.57 | 207 | 9.7 |
* Post-insolubilization (Second Dissolution Standards are used as insolubilization goals)
*The provided data is the result of laboratory testing performed according to internal testing methods. Corresponding performance at actual sites is not guaranteed.
- Insolubilization effect lasts for extended periods of time.
Acidic/Alkaline Additive Leach Testing *1 Example Results (100 kg/m3 of STA-M additive)
| Heavy metal/other | Soil dissolution (mg/L) |
Dissolution after insolubilization processing (mg/L) | ||
|---|---|---|---|---|
| Environmental Report 46 | Acidic additive | Alkaline additive | ||
| Lead | 5.7 | < 0.005 | < 0.005 | < 0.005 |
| Hexavalent chromium | 0.15 | 0.01 | 0.02 | 0.01 |
| Arsenic | 0.036 | < 0.002 | < 0.002 | < 0.002 |
| Fluorine | 1.4 | 0.05 | 0.05 | 0.07 |
| Boron | 0.65 | < 0.05 | < 0.05 | < 0.05 |
| Cyan | 1.0 | < 0.1 | < 0.1 | < 0.1 |
*1 "Relative Evaluation Method of Stability in pH Changes of Soil that Has Undergone Heavy Metal Insolubilization Treatment - Sulfuric Acid Additive Dissolution Testing Method/Slaked Lime Additive Dissolution Testing Method" from the Geo-Environmental Protection Center Technical Standards
*The provided data is the result of laboratory testing performed according to internal testing methods. Corresponding performance at actual sites is not guaranteed.
Case Study of Using STA-M On-site
Case Study of Using STA-M on Soil Highly Contaminated with Multiple Pollutants
- Plant site
- Amount of soil: 12,000 m3
- After pollutants are insolubilized to Second Dissolution Standards, soil is transported to controlled landfill sites.
| Concentration of Contamination in Soil | Concentration of Pollutants in Treated Soil | |||
|---|---|---|---|---|
| Heavy metal/other | Elution (mg/L) | Elution after insolubilization (mg/L) | ||
| 100kg/m3 | 150kg/m3 | |||
| Arsenic | 43.3 | 0.155 | 0.132 | |
| Lead | 2.33 | 0.015 | < 0.005 | |
| Cadmium | 0.067 | 0.015 | < 0.005 | |
| Mercury | 0.008 | 0.0005 | < 0.0001 | |
| Selenium | 0.371 | 0.045 | 0.015 | |
*The provided data is the result of laboratory testing performed according to internal testing methods. Corresponding performance at actual sites is not guaranteed.
Case Study of Insolubilization and Backfilling of Soil Contaminated with Multiple Pollutants
- Plant site
- Amount of soil: 550m3
- After pollutants are insolubilized to Second Dissolution Standards, soil is backfilled at original location.
| Concentration of Contamination in Soil | Concentration of Pollutants in Treated Soil | ||||
|---|---|---|---|---|---|
| Heavy metal/other | Elution (mg/L) | Elution after insolubilization (mg/L) | |||
| 60kg/m3 | 80kg/m3 | 100kg/m3 | |||
| Fluorine | 4.5 | 0.4 | 0.2 | 0.15 | |
| Lead | 0.20 | 0.007 | < 0.005 | < 0.005 | |
*The provided data is the result of laboratory testing performed according to internal testing methods. Corresponding performance at actual sites is not guaranteed.

Mixed with soil improver
Conceptual diagram of backfilling at original location
Appendix 1: Insolubilization Mechanism
Reaction 1: Solidification of Hydrotalcite-like Compounds
- A substance having a laminated structure formed by substituting a part of Mg2+ in Mg(OH)2, which is a hydration product of MgO, with Al3+.
- Water and anions are contained between the layers. Anions are exchanged with ions such as F-, B(OH)4-, CrO42-, AsO42-, SeO32- resulting in solidification.
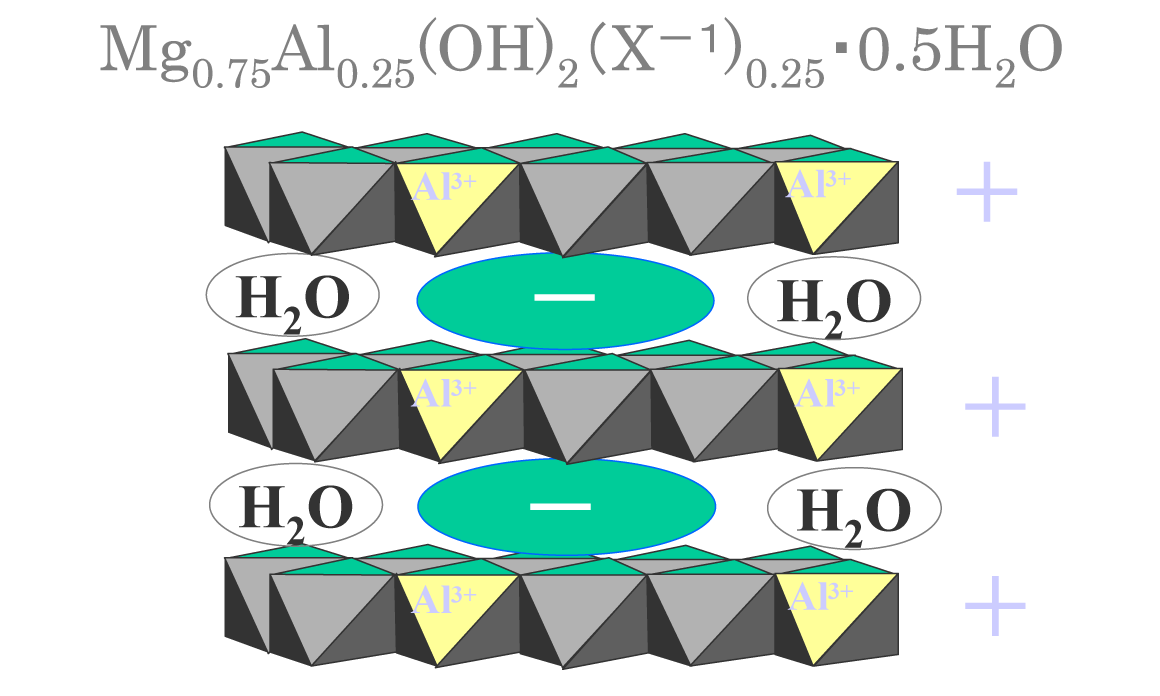
Conceptual Diagram Hydrotalcite Structure
Reaction 2: Insolubilization and Solidification through Formation of Magnesia Cement via MgO Hydration and Supply of Cl-
3MgO+MgCl2+nH2O→3MgO・MgCl2・nH2O
F and other anions electrically bind to the surface during the hydration reaction of MgO. Further immobilization occurs in the presence of MgCl2 resulting in solidification.
Reaction 3: Solidification via Dehydration by MgO Hydration and Carbonation
MgO+H2O→Mg(OH)2 Mg(OH)2+CO2→MgCO3
Adhesive effect of soil particles via basic magnesium carbonate produced by dehydration of soil water via MgO and carbonation using carbon dioxide gas in air.
Appendix 2: Solidification Effect
Solidification Effect - Compressive Strength after 28 Days of Curing
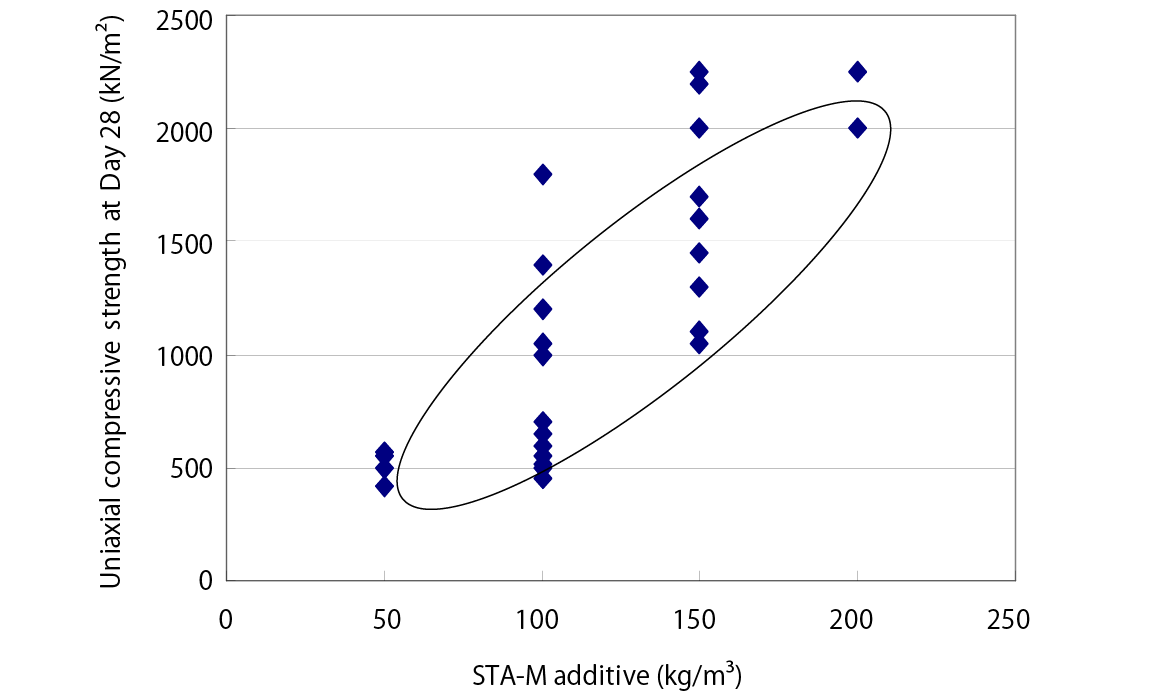
*The provided data is the result of laboratory testing performed according to internal testing methods. Corresponding performance at actual sites is not guaranteed.
TEL: +81-3-5445-5208 FAX: +81-3-5445-5220

